What is corrosion Inhibitor
A corrosion inhibitor is a substance or compound that is added to a fluid (usually a liquid) or a gas to decrease the rate of corrosion (the gradual deterioration of a material) that occurs on a metal surface. Corrosion is a natural process that can lead to the degradation and weakening of metals due to chemical reactions with the environment. Corrosion inhibitors work by forming a protective barrier on the metal surface, which either prevents the corrosive agents from coming into direct contact with the metal or alters the electrochemical reactions that drive the corrosion process.
Must Read : Hydraulic System
Types of Corrosion Inhibitor
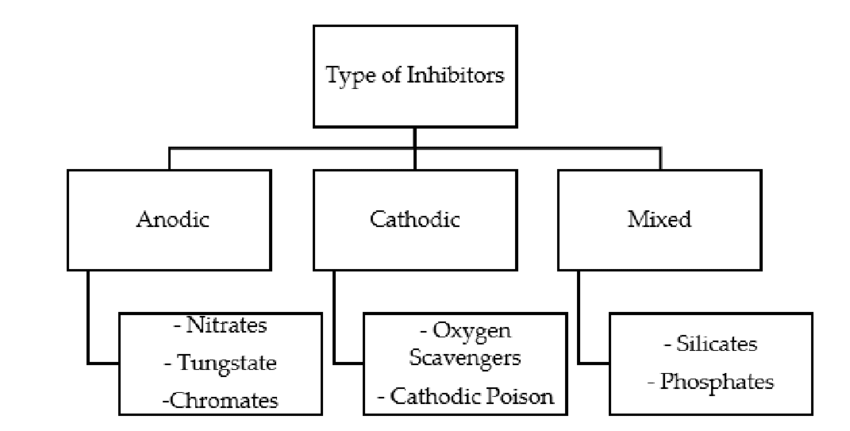
These some common types of corrosion inhibitors along with more details about each type:
1. Anodic Inhibitors:
Anodic inhibitors work by forming a protective oxide layer on the metal surface. This oxide layer acts as a barrier that prevents the metal from undergoing further oxidation and corrosion. Common anodic inhibitors include compounds like chromates, molybdates, and phosphates. These inhibitors are particularly effective for metals like aluminum and zinc.
2. Cathodic Inhibitors:
Cathodic inhibitors reduce the rate of cathodic reactions, which involve the reduction of oxygen or other species. By decreasing the availability of electrons required for these reduction reactions, the rate of corrosion is slowed down. Sodium nitrate, calcium nitrate, and other nitrates are examples of cathodic inhibitors. They are often used in closed systems like cooling water systems.
3. Mixed Inhibitors:
Mixed inhibitors work through a combination of both anodic and cathodic mechanisms. They provide comprehensive protection by reducing oxidation and reduction reactions simultaneously. Organic compounds containing nitrogen, sulfur, and oxygen atoms often function as mixed inhibitors. These compounds form protective films on metal surfaces.
4. Volatile Corrosion Inhibitors (VCIs):
VCIs are compounds that release volatile corrosion-inhibiting molecules into the surrounding environment. These molecules form a protective layer on metal surfaces, even in hard-to-reach areas. VCIs are commonly used in packaging materials for metal items, such as machinery parts or electronics, to prevent corrosion during storage and transportation.
5. Passivation Inhibitors:
Passivation inhibitors promote the passivation process, where a stable oxide layer forms on the metal surface. This oxide layer acts as a protective barrier against further corrosion. Passivation inhibitors often contain chemicals like chromates, which facilitate the formation of a stable and adherent oxide layer.
6. Film-Forming Inhibitors:
Film-forming inhibitors create a thin protective film on the metal surface. This film acts as a barrier that prevents corrosive substances from contacting the metal directly. These inhibitors are often used in applications where the metal is exposed to aggressive environments. Fatty acids, amines, and certain polymers are examples of film-forming inhibitors.
7. Inhibitors for Specific Environments:
Some corrosion inhibitors are formulated to be effective in specific environments. For example, in oil and gas industries, corrosion inhibitors may be tailored to withstand the acidic and high-pressure conditions found in pipelines and drilling equipment. In marine environments, inhibitors might be designed to combat the corrosive effects of saltwater.
It’s important to note that the selection of a corrosion inhibitor depends on factors such as the type of metal, the corrosive agents present in the environment, the operating conditions, and the intended application. Additionally, while corrosion inhibitors can significantly reduce the rate of corrosion, they may not eliminate it entirely. Regular monitoring and maintenance are still important to ensure long-term protection against corrosion.
example of corrosion inhibitor
Certainly, here are a few examples of corrosion inhibitors along with their applications:
1.Chromate-based Inhibitors:
- Example: Sodium Chromate (Na2CrO4)
- Application: Used as an anodic inhibitor in cooling water systems, aircraft components, and certain coatings to form a protective chromium oxide layer on the metal surface.
2.Zinc-based Inhibitors:
- Example: Zinc Sacrificial Anode
- Application: Used in marine environments, pipelines, and underground structures to protect other metals through galvanic corrosion. Zinc corrodes preferentially, sacrificing itself to protect the more valuable metal.
3.Molybdate-based Inhibitors:
- Example: Sodium Molybdate (Na2MoO4)
- Application: Used in cooling water systems, boilers, and heat exchangers to inhibit both anodic and cathodic corrosion processes by forming a protective film on metal surfaces.
4.Volatile Corrosion Inhibitors (VCIs):
- Example: VCI Paper
- Application: Used for protecting metal components during storage and transportation. VCIs in the paper release vapor that forms a thin, protective layer on metal surfaces, preventing corrosion.
5.Nitrite-based Inhibitors:
- Example: Sodium Nitrite (NaNO2)
- Application: Used to protect ferrous metals in closed-loop cooling systems by promoting the formation of a passive iron oxide layer on the metal surface.
6.Organic Film-Forming Inhibitors:
- Example: Fatty Acid Corrosion Inhibitors
- Application: Used in closed systems like pipelines and hydraulic systems. Fatty acids form a protective film on metal surfaces, reducing the contact between the metal and corrosive agents.
7.Phosphate-based Inhibitors:
- Example: Sodium Phosphate (Na3PO4)
- Application: Used in cooling water systems to form a protective scale on metal surfaces, reducing both anodic and cathodic corrosion reactions.
8.Amine-based Inhibitors:
- Example: Cyclohexylamine
- Application: Used in steam condensate systems and boilers to form a protective film on metal surfaces and maintain an alkaline pH, reducing corrosion rates.
9.Aluminum-based Inhibitors:
- Example: Aluminum Sacrificial Anode
- Application: Similar to zinc, aluminum sacrificial anodes are used to protect pipelines, tanks, and marine structures by corroding in preference to the protected metal.
10.Silicate-based Inhibitors:
- Example: Sodium Silicate (Na2SiO3)
- Application: Used in concrete admixtures to protect embedded reinforcement bars from corrosion caused by chloride ions.
11. Copper-based Inhibitors:
- Example: Copper-based Algaecides
- Application: Used in cooling water systems to inhibit microbial growth, which can contribute to corrosion and fouling.
It’s important to note that the selection of a corrosion inhibitor depends on the specific application, the type of metal, the environment, and regulatory considerations. Additionally, the effectiveness of corrosion inhibitors can vary based on factors such as concentration, temperature, and exposure time.
Mechanisms of Corrosion Inhibitors
Corrosion inhibitors work through various mechanisms to protect metal surfaces from undergoing corrosion. These mechanisms are based on altering the electrochemical reactions that drive the corrosion process. Here are some key mechanisms through which corrosion inhibitors operate:
- Adsorption Mechanism:
Corrosion inhibitors often function by adsorbing onto the metal surface, forming a protective barrier. This barrier physically separates the metal from the corrosive environment, preventing direct contact with corrosive agents. The adsorption can occur through chemical bonding or weak interactions between the inhibitor molecules and the metal surface. - Anodic Inhibition:
Anodic inhibitors reduce the oxidation (anodic) reaction that occurs at the metal surface. They achieve this by forming a passivating oxide layer that hinders the release of metal ions and prevents the propagation of corrosion reactions. - Cathodic Inhibition:
Cathodic inhibitors work by slowing down the reduction (cathodic) reactions that involve the consumption of electrons and the reduction of oxidants (like oxygen). By inhibiting these cathodic reactions, the flow of electrons necessary for corrosion is reduced. - Mixed Inhibition:
Mixed inhibitors combine aspects of both anodic and cathodic inhibition. They act to reduce both oxidation and reduction reactions, effectively slowing down the entire corrosion process. - Formation of Protective Films:
Some inhibitors facilitate the formation of thin, protective films on the metal surface. These films can be composed of oxides, hydroxides, or other compounds that act as a barrier against corrosive agents. - pH Modification:
Certain inhibitors modify the pH of the environment around the metal surface. By shifting the pH to less corrosive values, these inhibitors can slow down the corrosion reactions. - Complexation:
Inhibitors can form complexes with metal ions, preventing their participation in corrosion reactions. This effectively reduces the rate of metal dissolution. - Volatility and Vapor Phase Inhibition:
Volatile corrosion inhibitors (VCIs) release vapor-phase inhibitors that condense on metal surfaces, forming a protective layer. These molecules inhibit corrosion by creating a barrier against corrosive agents. - Passivation Enhancement:
Some inhibitors promote the natural passivation process, where a stable oxide layer forms on the metal surface. This layer acts as a barrier against further corrosion. - Ion Dissolution Inhibition:
Certain inhibitors work by hindering the dissolution of metal ions into the surrounding solution. This reduces the rate of metal loss and the progression of corrosion.
The effectiveness of a corrosion inhibitor depends on factors such as the inhibitor’s chemical properties, concentration, the type of metal, the corrosive environment, and the intended application. Different types of inhibitors may be more suitable for specific conditions or types of corrosion. It’s essential to select the appropriate inhibitor mechanism based on the specific scenario to achieve effective corrosion protection.
Application of Corrosion Inhibitor
Corrosion inhibitors are used in various industries and applications to prevent or mitigate the effects of corrosion on metal surfaces. Here are some common applications of corrosion inhibitors:
- Oil and Gas Industry:
Corrosion inhibitors are extensively used in the oil and gas sector to protect pipelines, storage tanks, and equipment from corrosion caused by aggressive fluids, water, and gases present in oil and gas production, transportation, and refining processes. - Water Treatment:
In cooling water systems, boilers, and other water-related industrial equipment, corrosion inhibitors help prevent the degradation of metal surfaces due to the presence of water and dissolved ions. This is crucial to maintain the efficiency and longevity of equipment. - Aerospace and Aviation:
Corrosion inhibitors are used to protect aircraft structures, components, and engines from corrosion caused by exposure to harsh environmental conditions, including moisture, salt, and pollutants in the air. - Automotive Industry:
In automotive applications, corrosion inhibitors are used to protect vehicles’ undercarriages, body panels, and other metal parts from corrosion due to road salts, moisture, and environmental exposure. - Marine Industry:
Ships, offshore platforms, and marine equipment are exposed to highly corrosive seawater. Corrosion inhibitors are used to extend the lifespan of these assets by preventing or slowing down the corrosion process. - Metal Processing and Manufacturing:
During metal processing and manufacturing, corrosion inhibitors can be applied to protect metal workpieces, machinery, and tools from rusting and other forms of corrosion during storage, transportation, and machining operations. - Infrastructure and Construction:
Corrosion inhibitors are used in the construction industry to protect steel reinforcements in concrete structures like bridges, buildings, and highways. They help extend the service life of these structures by reducing the risk of corrosion-induced deterioration. - Mining Industry:
Mining equipment and infrastructure can be exposed to corrosive environments due to the presence of chemicals and moisture. Corrosion inhibitors can help protect these assets and maintain their operational integrity. - Electronics and Electrical Systems:
Corrosion inhibitors can be used to protect electrical connections, terminals, and components from corrosion, ensuring the reliable operation of electronic devices and systems. - Metal Preservation and Storage:
Corrosion inhibitors are employed to protect metal items during storage and transportation. They are commonly used in packaging, such as vapor-phase inhibitors, which release protective molecules to create a barrier against corrosion. - Nuclear Industry:
In the nuclear sector, corrosion inhibitors are used to protect metal components and equipment from the effects of radiation, heat, and chemically aggressive environments. - Chemical Processing:
In chemical plants, corrosion inhibitors are used to safeguard metal equipment and pipelines from the corrosive effects of chemicals being processed or transported.
The specific corrosion inhibitor and application method chosen depend on factors such as the type of metal, the corrosive agents present, the operating environment, regulatory requirements, and economic considerations. Corrosion inhibitors play a critical role in maintaining the integrity and functionality of various industries and their assets.
Advantages of Corrosion Inhibitor
Corrosion inhibitors offer several advantages in various industrial applications due to their ability to prevent or mitigate the effects of corrosion on metal surfaces. Some of the key advantages of using corrosion inhibitors include:
- Extended Equipment Lifespan: Corrosion inhibitors help protect metal equipment, structures, and components from degradation, thus extending their operational lifespan. This reduces the need for frequent replacements and repairs, leading to cost savings over time.
- Reduced Maintenance Costs: By minimizing the occurrence of corrosion-related damage, corrosion inhibitors can significantly reduce maintenance and repair costs. Equipment downtime and associated labor costs are also minimized, leading to increased operational efficiency.
- Preservation of Asset Value: Corrosion inhibitors help maintain the value of assets, such as vehicles, machinery, and infrastructure. This is particularly important in industries where the value of assets directly impacts the financial health of the business.
- Enhanced Safety: Corrosion-induced failures can lead to accidents and safety hazards. By preventing corrosion-related structural integrity issues, corrosion inhibitors contribute to a safer working environment for employees and the public.
- Improved Performance: Corrosion inhibitors can help maintain the performance and efficiency of equipment and systems by preventing the buildup of corrosion-related deposits that can hinder fluid flow, heat transfer, and other essential processes.
- Environmental Protection: Some corrosion inhibitors are formulated to be environmentally friendly and comply with regulations. By preventing corrosion and reducing the need for maintenance and replacement, they can contribute to sustainability efforts by minimizing waste and resource consumption.
- Flexibility in Design: Corrosion inhibitors can provide design flexibility by allowing the use of materials that might otherwise be susceptible to corrosion. This can lead to more innovative and cost-effective designs in various industries.
- Mitigation of Corrosion-Induced Contamination: Corrosion of metal surfaces can lead to contamination of fluids and products, particularly in industries like food and pharmaceuticals. Corrosion inhibitors help maintain the quality and purity of products by preventing metal-related contaminants.
- Ease of Application: Many corrosion inhibitors are available in various forms, such as liquids, powders, and coatings, making them relatively easy to apply to different types of surfaces and equipment.
- Customization: It can be tailored to specific applications, environments, and metals. This allows for customized solutions that address the unique corrosion challenges of each industry or situation.
- Protection During Storage and Transportation: Corrosion inhibitors can safeguard metal items during storage and transportation, ensuring that products reach their intended destinations in good condition.
- Compatibility: It is often designed to be compatible with various materials and coatings, allowing for their use alongside other protective measures without causing adverse effects.
- Ease of Monitoring: Some corrosion inhibitors come with monitoring systems that allow for the assessment of their effectiveness over time, enabling timely adjustments if necessary.
It’s important to note that while corrosion inhibitors offer numerous benefits, their selection, application, and monitoring should be performed carefully to ensure optimal performance and to address any potential side effects or environmental concerns.
Disadvantages of Corrosion Inhibitor
While corrosion inhibitors offer various advantages, there are also some potential disadvantages and limitations associated with their use. It’s important to consider these factors when deciding whether to use corrosion inhibitors in a specific application. Some disadvantages of corrosion inhibitors include:
- Environmental Concerns: Some corrosion inhibitors, particularly those containing toxic or environmentally harmful substances like chromates, can pose risks to the environment during manufacturing, usage, and disposal. Regulations and sustainability considerations may limit the use of certain inhibitors.
- Health and Safety Risks: Certain corrosion inhibitors, especially those with toxic components, can pose health and safety risks to workers during handling and application. Adequate safety measures and protective equipment are necessary to minimize these risks.
- Inhibitor Compatibility: Corrosion inhibitors may not be compatible with all materials, coatings, and fluids. Incompatibility could lead to adverse chemical reactions, reduced effectiveness, or even equipment damage.
- Effectiveness Over Time: The effectiveness of some corrosion inhibitors may diminish over time due to factors like depletion, degradation, or changes in the environment. Regular monitoring and maintenance are necessary to ensure consistent protection.
- Cost: It can be costly, particularly when required in large quantities or for continuous application. The initial investment, ongoing monitoring, and potential need for frequent reapplication contribute to the overall cost.
- Limited Longevity: Some corrosion inhibitors may provide only temporary protection, requiring frequent reapplication. This can be a logistical challenge and increase maintenance efforts.
- Interference with Processes: In certain applications, It might interfere with the intended processes, such as heat transfer, fluid flow, or electrical conductivity. This interference could reduce system efficiency.
- Complex Application: The proper application of corrosion inhibitors requires expertise to ensure that the inhibitor is evenly distributed and adheres well to the metal surface. Improper application can lead to uneven protection and reduced effectiveness.
- Residue Buildup: In some cases, the use of corrosion inhibitors can lead to the buildup of residues on metal surfaces, which may affect the equipment’s performance or require additional cleaning steps.
- Limited Coverage: It might not provide uniform protection on complex or hard-to-reach surfaces, leaving certain areas vulnerable to corrosion.
- Resistance Development: Over time, some corrosive environments can adapt to the presence of inhibitors, potentially leading to the development of resistance and reduced inhibitor effectiveness.
- Regulatory Compliance: The use of certain corrosion inhibitors might be restricted or subject to regulations due to environmental or health concerns, necessitating careful consideration and adherence to guidelines.
- Effect on Coatings: It might affect the performance or adhesion of certain coatings, which could impact the overall protection of the metal surface.
- Unforeseen Side Effects: In some cases, It might have unexpected side effects or interactions with other substances in the environment, leading to unintended consequences.
When considering the use of corrosion inhibitors, it’s important to conduct a thorough evaluation of the specific application, environment, materials, costs, and potential risks to determine whether the benefits outweigh the disadvantages in a given situation.
Reference : https://en.wikipedia.org/wiki/Corrosion_inhibitor